





Published on Sep 05, 2023
The objective: Which method of creating cellulosic ethanol through mature lignocellulosic material would be the most effective and feasible in terms of timeline, cost, and procedure: cellulolysis using cellulase and fermentation or the gasification method using fermentation?
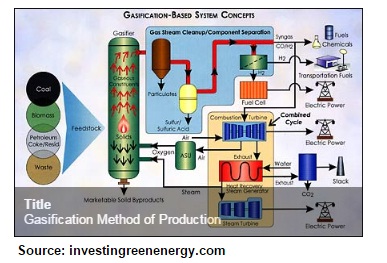
I believe that the most effective method of creating cellulosic ethanol would be the cellulolysis procedure, as it is natural, self-acting, and does not produce harmful gases. Simply, through this process as complex cellulose is broken into sugars and it then fermented to produce ethanol. In comparison to the gasification method, this proves to be much safer as it does not produce carbon monoxide and hydrogen to catalyze, which can be of potential danger.
• 100 mL of water
• 0.7 g of cellulase enzyme
• 3 g of cellulose
• 5 mL of aqueous copper (II) sulfate (Fehlings A)
• 5 mL of aqueous colourless solution of potassium sodium tartrate and sodium hydroxide
• 5 g of cellulose
• 1 g of yeast
• 100 mL of limewater
• 1 small test tube
• 1 thermometer
• laboratory distillation equipment (including a hot plate, distillation and receiving flasks, and a magnetic stirrer
• temperature controlled flask for glucose production
1. Took 30ml solution of 0.7% enzyme cellulase in water (0.7g in 100ml).
2. Added 3 g cellulose in it.
3. Heated the mass to 50°C and maintained for 3 hrs under slow stirring.
4. The stirring was then stopped and the mass was allowed to settle.
5. The upper aqueous layer of liquid was removed (15ml).
6. Fresh 15ml water was added and stirring was continued for next 3hrs.
7. The 15ml solution was removed was nothing but the syrup solution i.e. glucose in water.

This report summarizes how my innovation proved to support my hypothesis and effectively worked to reach the goal I hoped for. In addition, it will outline various reasons for occurrences during the experiment and relate back to the preliminary research in each stage of my experiment.
In conclusion, I believe that I effectively supported my hypothesis by carrying out various experiments in order to reach the end result of creating biodegradable ethanol that can be converted to environmentally friendly biofuels, simply from the starting point of fiber and cellulose based paper towel, through the aid of the cellulose enzyme. Thus, through this experiment, I went through a multi-step process, known as enzymatic hydrolysis that converts paper towels (fiber), into glucose, to ethanol and finally, in theory, to biofuel/bioethanol. In hypothesis, I claimed that this method of converting fiber to biofuels is the most efficient compared to other models, mainly due to the low cost, time efficiency, as well as the readily available, easily achieved materials and reasonable time- bound, swift process.
The most obvious in my analysis was that the temperature of the water added to create a distillation mass to produce glucose was not controlled, even though the temperature of the entire mass was controlled. This would have led to adverse effects, as heated water would act differently than colder water when added to the mass. Thus, this would have led to different result, such as the cellulase and cellulose dissolving in a different time frame or quality. In addition, this was a systematic error as it was resultant of a flaw in the procedure, which could have been meticulous. This, obviously, would have been avoided if the temperature of the water added was monitored and controlled throughout the experiment, therefore resulting in further precise outcomes
In the primary part of my experiment, while converting cellulose to glucose, it was stated in the procedure that the mass must be heated to 50o C, however, due to the limitations of the machine itself, it often drifted 1-2 o C away. Though this did not greatly affect my experiment, it would have proved to have an effect if the experiment lasted longer or was completed more than twice. This, error, if it had to be fixed, would be most easily mended by renewing and renovating the temperature control system or to simply use a variating temperature in the procedure itself to account for the machine.
One final error that was committed was the lack of recognizing physical variation, which is the act of carrying out multiple experiments in order to detect variances or problems in the experiment that went undetected at other times. I completed this experiment only twice, thereby; I did not have the opportunity to average out values and effects on my experiment more than once. Thus, if I had completed the experiment in further extremity, as a solution, I would have arrived at more accurate results and data to support my hypothesis.